sliceGolgi Kit (Cat#003760)
USD $483 / sliceGolgi Kit + USD $136 / Fixative Kit
(Distributor and quantity discounts available, please send an email to
contact@bioenno.com to order.
For common questions and answers, please check our FAQ.)
Perform Golgi-staining on Free-floating Slices/Sections
Bioenno also offers the sliceGolgi Kit, with an additional Fixative Kit. The sliceGolgi Kit is designed to run Golgi-staining on slices/sections. The impregnation and staining can be performed on free-floating sections (50~400 micron thickness). The additional Fixative Kit uses Bioenno’s aldehyde to process additional samples. These kits have been extensively tested across both rat and mice brain sections and consist of:
- Freshly harvested sections, such as acute slices quickly prepared using tissue chopper.
- Organotypic slice cultures, e.g. cultured hippocampal slices and cultured cortical slices.
- Artificial cerebrospinal fluid (ACSF)-infused slices that have completed electrophysiological recording and drug administration.
- Sections derived from brains fixed with Bioenno aldehyde Fixative (Catalog Number: 003780) via either perfusion or immersion. The fixed brains can be systematically sliced at 50~400 microns at room temperature (RT, 15℃ – 25℃), and the sections can be subjected to Golgi staining alone, immunohistochemistry/immunocytochemistry (ICC) alone, or combined Golgi staining and ICC.
- Additional Fixative Kit allows for processing of more samples. Bioenno suggests purchasing the sliceGolgi Kit along with the Fixative Kit.
The sliceGolgi Kit may be applied to retinal, spinal cord, and cultured cells to stain the neuronal processes.
The sliceGolgi Kit yields both reliable and high quality labeling of dendrites and spines (see Figures). The impregnation of neurons in 50 to 200 micron thick sections is super-fast, only 2-3 days in some cases. Generally, impregnation will take 4-7 days depending on the age and thickness of the sections. The kit can be stored in a dark area at RT for up to 12 months.
Combined Golgi-staining and immunohistochemistry/immunocytochemistry (ICC)
Bioenno Tech has successfully combined the use of the sliceGolgi Kit with ICC on the same brain section (see Figures 4-9). To perform the combined Golgi staining and ICC, fixed sections are first subjected to Golgi staining, and then to ICC:
(1) Tissue Handling: Animals are intracardially perfused with saline followed by a fixative (Catalog Number: 003780) developed by Bioenno Tech. Brain are dissected from the skull and post-fixed for 1-3 days at 4ºC. Brains are then sectioned at 50~100 micron thickness using a vibratome or similar type of microtome/microslicer at RT. Sections are collected in 0.1M PB (pH 7.4) and can be stored in the buffer for up to one week at 4ºC.
The fixed sections can be used for Golgi staining alone, ICC alone, and combined Golgi staining and ICC.
(2) Golgi Staining: Free-floating sections are subjected to Golgi staining using the sliceGolgi Kit at RT. Impregnation of neurons may take 2-5 days.
(3) ICC: Free-floating sections can be subjected to standard avidin-biotin complex (ABC) methods. The immunoreaction product can be visualized by incubating sections in 3,3′-diaminobenzidine (DAB) containing H2O2.
Note: The sliceGolgi Kit does not work for frozen tissues. Fixed tissue block cannot be frozen prior to cutting.
Product Features
- Suitable for sliced/sectioned tissues (50 to 400 microns thick)
- Perform impregnation and staining on free-floating sections
- Novel aldehyde fixative and enhanced impregnation solution
- Impregnation of neurons is fast (2-7 days)
- Combines both Golgi-staining and immunohistochemistry
- Proven results/user friendly
- Sufficient for up to 1,000 brain sections (50-400 microns thick with dimensions of ~1 x 1.5 cm)
- For in-vitro lab use
- Dedicated technical support
- 12-month warranty
Product Description and Protocol
Proven Results Using the sliceGolgi Kit (Catalog Number: 003760)
Dendritic branches and spines have been reliably impregnated and stained with the sliceGolgi Kit.

Fig. 1 – An impregnated and stained neuron in layer II of the frontal cortex
The sliceGolgi Kit was used to impregnate and stain the neuron in the frontal cortex of a postnatal day 12 (P12) C57BL mouse. Dendritic spines (arrows) of a neuron in a 200 micron-thick section were well labeled. Arrowheads indicate filopodia-like protrusions, the immature spines. The image was taken using a 20x objective lens.

Fig. 2 – Dendritic branches of a pyramidal neuron in layer III of the neocortex
The sliceGolgi Kit was used to impregnate and stain the oblique dendrites and main branch (stem) of a pyramidal neuron located in the motor area of the frontoparietal cortex at 20x objective. The C57BL mouse is 12 days old.
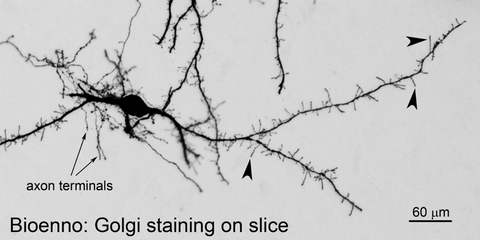
Fig. 3 – A neuron in the lateral hypothalamus
The sliceGolgi Kit was used to impregnate neurons in 100 µm-thick sections under free floating condition. The stained neuron with axon terminals was taken (20x objective lens) from the lateral hypothalamic area of a P12 C57BL mouse. Numerous filopodia-like protrusions (arrowheads) were found on the dendritic branches.
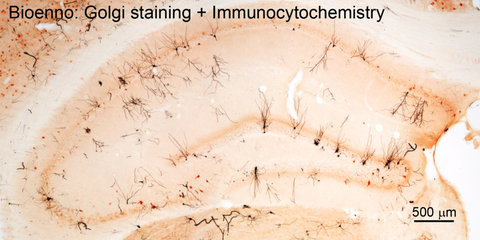
Fig. 4 – The combined use of sliceGolgi Kit and immunohistochemistry / immunocytochemistry (ICC)
The combination of Golgi staining and ICC allows simultaneous visualization of dendritic spines and immunoreactive products. Generally, free-floating sections were first processed for Golgi-staining, and then for ICC. For Golgi staining, animals were perfused via the ascending aorta with 0.9% saline solution followed by freshly prepared fixative (Catalog Number: 003780, Bioenno Tech). Brains were dissected from the skull and postfixed for 1-3 days (4ºC). Brains were then sectioned at 50-100 μm thickness using a vibratome, and sections were collected into tissue-culture wells in 0.1 M PB (pH 7.4). The sliceGolgi Kit (Catalog Number: 003760) was used to impregnate (2-5 days, RT) and stain the dendrites (black). To perform ICC, free-floating sections were subjected to standard avidin-biotin complex (ABC) methods. The immunoreaction product was visualized by incubating sections for 8-10 minutes in 0.04% 3,3′-diaminobenzidine (DAB) containing 0.01% H2O2. The image was taken from the hippocampus of a 2-month old C57 mouse using a 4x objective lens.

Fig. 5 – Golgi staining and ICC performed on the same brain section
Combined Golgi staining and ICC: 1) C57 mouse was intracardially perfused with saline followed by a Bioenno-developed aldehyde fixative (Catalog Number: 003780). 2) Free-floating sections (50 microns) were subjected to Golgi staining using the sliceGolgi kit. The time of impregnation was 2 days only at 22 ± 1ºC. 3) ICC for parvalbumin (PV) was processed using standard avidin-biotin complex methods. The incubation time of monoclonal mouse anti-PV antibody (1:20,000, MAB1572, Chemicon) was overnight at 22 ± 1ºC. The image was taken from the thalamus using a 4x objective lens.

Fig. 6 – Dendritic branches and PV-immunoreactive neurons
Golgi staining and ICC were performed on the same brain section. Golgi staining was performed on free-floating sections (100 µm) using the sliceGolgi Kit. The impregnation time was 3 days at 22 ± 1ºC. The image was taken (63x objective) from the subiculum of a 4-month old C57 mouse.

Fig. 7 – Golgi-stained dendrites and immuno-labeled neurons
The sliceGolgi Kit was used to impregnate and stain the dendritic branches of a cortical neuron in layer III of the cortex. The immunoreactive neurons were visualized in the same area. The image was taken (63x objective) from a 4-month old C57 mouse.

Fig. 8 – Golgi-stained cortical neurons and immuno-labeled neurons
The sliceGolgi Kit was used to impregnate and stain the dendritic branches of neurons in the deeper layer of cortex. The impregnation of neurons in the 100 µm-thick sections took 2 days only at 22 ± 1ºC. The immunoreactive neurons were visualized in the same area. The image was taken from a 2-month old C57 mouse using a 20x objective lens.

Fig. 9 – Golgi staining and ICC performed on the same brain section
The sliceGolgi Kit was used to label dendritic branches. The boxed areas in the top panel, shown at 20x objective, were magnified (63x) to highlight the dendritic spines (arrows) and immuno-labeled neurons (arrowheads). These images were taken from the frontoparietal cortex (somatosensory area) of a 2-month-old C57BL mouse.
FAQ about SliceGolgi Kits
· I am using the sliceGolgi Kit. How long can the tissue be kept in the Fixative?
The fixative provided in the kit or from an additional Fixative Kit (cat #: 003780) affects the staining of neurons. Long time exposure (days to weeks) to the Fixative will result in numerous axons to be stained, which will hamper the labeling of dendritic spines.
If the animals are perfused with the Fixative, post-fix is generally not necessary. If the tissue must be post-fixed, post-fix the tissue as short as possible.
· I am using the sliceGolgi Kit. Can I perform the impregnation on the Bioenno Fixative-fixed tissue blocks?
Yes. The impregnation can be performed on either tissue blocks or free-floating sections. If you plan to run the impregnation on tissue blocks instead of on sections, please rinse the tissue blocks in dH2O for a couple of hours (change dH2O several times) to remove the extra fixative, and then use the impregnation solution.
· How to apply the sliceGolgi kit on organotypic slice cultures?
Take out the cultures from a CO2 incubator, rinse the membrane (the cultures are seated on the membrane) for seconds with 0.1 M PB at room temperature (rinsing in cold buffer will result in the retraction of spines), and immerse the membrane in the kit provided fixative. Generally, exposure to the Fixative for 2-4 hrs will be good for ~350 µm slice. Long-term fixation such as overnight or days may increase the labeling of neuronal axons.
· How to use the sliceGolgi Kit to perform combined Golgi staining and immunohistochemistry (IHC or ICC)?
The fixed sections are first subjected to Golgi staining under free-floating conditions, and then to ICC. To make the combined reaction successful, fix the tissues as short as possible and complete the impregnation of neurons in 2~3 days, because long term exposure to fixative and/or impregnation solution will hamper the epitopes of many antigens. In addition to performing a good Golgi staining, the avidin-HRP detection system for ICC should also be very sensitive. The Bioenno DAB-Kit and DAB-Co Kit work very well under the following procedures:
1) Perfuse animals with saline followed by a Bioenno Fixative (500ml provided in the kit; or from Fixative Kit, Cat 003780) for 20~25 min. The brain is dissected from the skull and stored in 0.1M PB (pH 7.4), followed by vibratome slicing (~50 microns) at room temperature (~22 ºC). Sections can be collected in 0.1M PB (pH 7.4). If the perfusion is not good, post-fix the tissue for 1~3 hours.
2) Free-floating sections are subjected to Golgi staining using the sliceGolgi Kit at RT. Complete the impregnation within 2~3 days. Sections are briefly washed in 0.01 M PBS-T, followed by the staining (2~4 min) and post-staining (~1 min). If the impregnation of neurons required 5~7 days, the following ICC will be very hard.
3) Wash the section in PBS-T for minutes, and then H2O2 (30 µl original 30% H2O2 into 10 ml PBS-T) for 20 min, followed by PBS-T washing.
4) Perform standard ICC: incubate the sections in primary antibody overnight, wash in PBS-T for 10~15 min, and then biotin-conjugated second antibody and avidin-biotin complex. The immunoreaction products can be visualized by incubating sections in DAB or DAB-cobalt containing H2O2 (e.g., Bioenno DAB or DAB-Co Kit).
If you used a biotin-labeled primary antibody, the biotin-conjugated second antibody described above can be ignored.
5) Mount, air-dry, clear the sections as usual. Coverslip with Permount® mounting medium.
Some Publications cited with SliceGolgi Kits
(For more detail, please see Publication List)
-
Running-induced memory enhancement correlates with the preservation of thin spines in the hippocampal area CA1 of old C57BL/6 mice
Benke Xu, Anbang Sun, Yun He, Feng Qian, Lian Liu, Yuncai Chen, Huanmin Luo(2017), Neurobiology of Aging,52, 106-116
Link: http://www.sciencedirect.com/science/article/pii/S0197458017300027 -
Enduring memory impairments provoked by developmental febrile seizures are mediated by functional and structural effects of neuronal restrictive silencing factor
Katelin P Patterson, Jeremy M Barry, Megan M Curran, Akanksha Singh-Taylor, Gary Brennan, Neggy Rismanchi, Matias Page, Yoav Noam, Gregory L Holmes and Tallie Z Baram(2017), Journal of Neuroscience,3748-16
Link: http://www.jneurosci.org/content/early/2017/03/08/JNEUROSCI.3748-16.2017
-
Converging, Synergistic Actions of Multiple Stress Hormones Mediate Enduring Memory Impairments after Acute Simultaneous Stresses
Yuncai Chen, Jenny Molet, Julie C. Lauterborn, Brian H. Trieu, Jessica L. Bolton, Katelin P. Patterson, Christine M. Gall, Gary Lynchand Tallie Z. Baram (2016), Journal of Neuroscience. 36 (44), 11295-11307
Link: http://jneurosci.org/content/36/44/11295.abstract?etoc -
Dual Function of NRP1 in Axon Guidance and Subcellular Target Recognition in Cerebellum
Ludovic Telley, Christelle Cadilhac, Jean-Michel Cioni, Alexandre Dayer, Andrea B. Huber, Fabrice Ango (2016), Neuron,91 (6), 1–16
Link: http://www.cell.com/cms/attachment/2064169883/2065881213/mmc2.pdf -
Foxp2 controls synaptic wiring of corticostriatal circuits and vocal communication by opposing Mef2c
Yi-Chuan Chen, Hsiao-Ying Kuo, Ulrich Bornschein, Hiroshi Takahashi, Shih-Yun Chen, Kuan-Ming Lu, Hao-Yu Yang, Gui-May Chen, Jing-Ruei Lin, Yi-Hsin Lee, Yun-Chia Chou, Sin-Jhong Cheng, Cheng-Ting Chien, Wolfgang Enard, Wulf Hevers, Svante Pääbo, Ann M Graybiel & Fu-Chin Liu (2016). Nature Neuroscience,19, 1513–1522
Link: http://www.nature.com/neuro/journal/v19/n11/full/nn.4380.html
-
Diversity of Reporter Expression Patterns in Transgenic Mouse Lines Targeting Corticotropin-Releasing Hormone-Expressing Neurons.
Yuncai Chen, Jenny Molet, Benjamin G. Gunn, Kerry Ressler, Tallie Z. Baram (2015). Endocrinology, 156(12), 4769-4780.
Link:http://www.ncbi.nlm.nih.gov/pubmed/26402844
-
Genetic rescue of CB 1 receptors on medium spiny neurons prevents loss of excitatory striatal synapses but not motor impairment in HD mice.
Naydenov, A. V., Sepers, M. D., Swinney, K., Raymond, L. A., Palmiter, R. D., & Stella, N. (2014). Neurobiology of Disease, 71, 140-150.
Link: http://www.ncbi.nlm.nih.gov/pubmed/25134728
brain tissue, dendrite, dendritic spine, GFP, gfp, golgi cox, golgi impregnation, golgi stain, golgi’s method, impregnation, rapid golgi, spine, staining, super golgi, supergolgi, synapse






